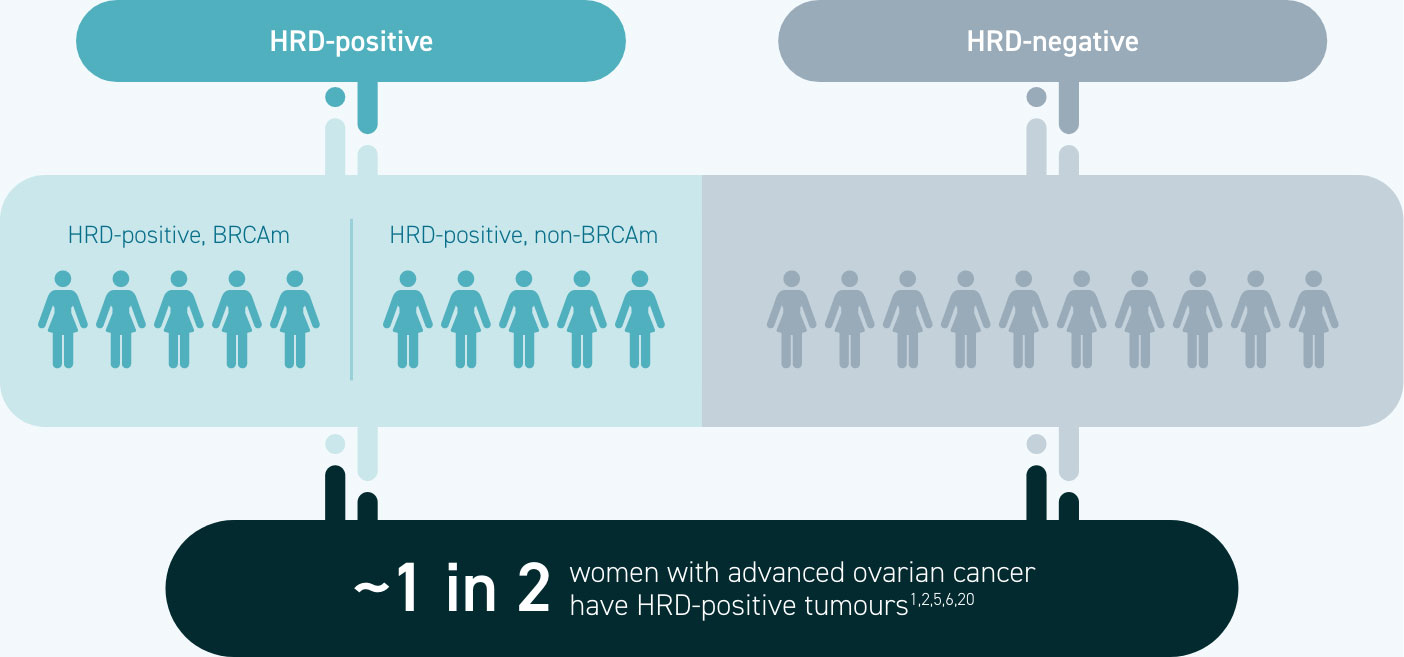
Approximately half of women with epithelial ovarian cancer have homologous recombination deficient (HRD) tumours.1,2
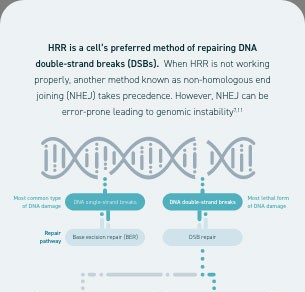
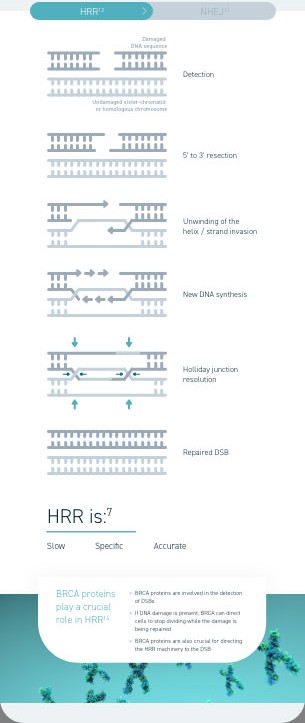
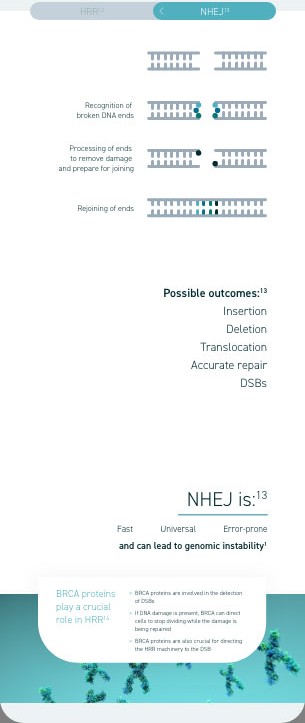
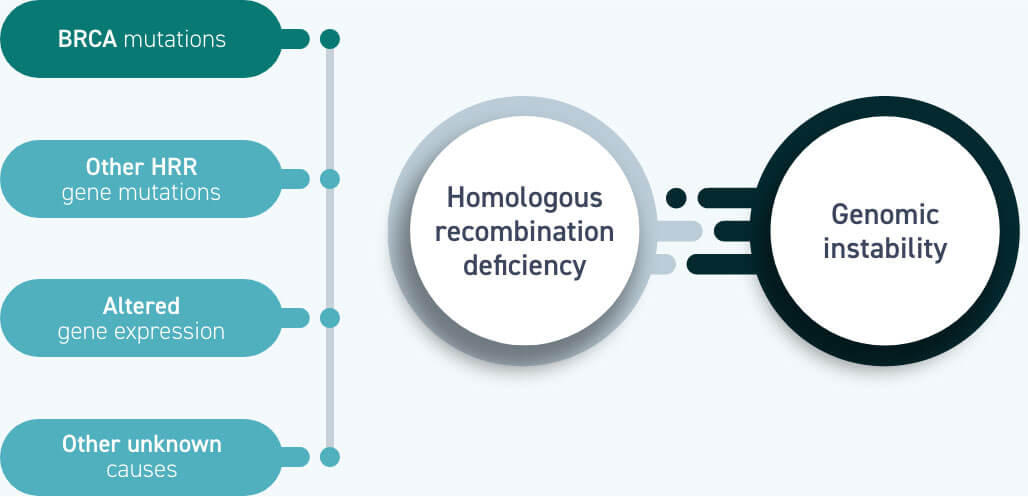
Homologous recombination deficiency is a phenotype of a cell or tumour that has an impaired ability to perform homologous recombination repair (HRR; a cell’s preferred method of repairing DNA double-strand breaks [DSBs]).1
Ovarian tumours with homologous recombination deficiency represent a group of patients who normally have an increased sensitivity to platinum-based chemotherapy and poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors.1,3–6
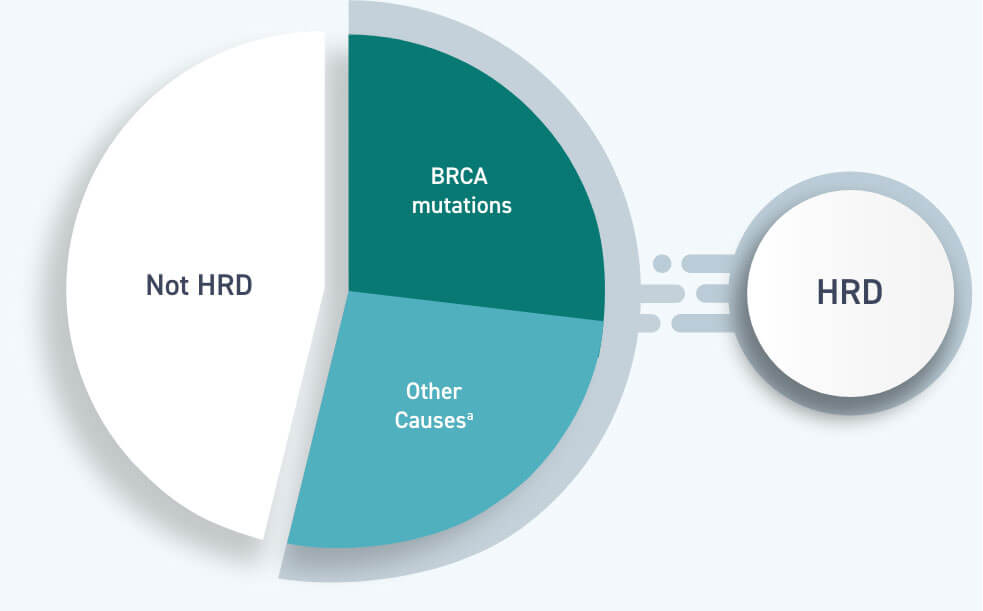
Homologous recombination deficiency is present in ~50% of epithelial ovarian cancers1,5,6
Homologous recombination deficiency is a phenotype of a cell or tumour that has an impaired ability to accurately repair DNA7
Research has shown that homologous recombination deficiency in ovarian cancer may be associated with improved responses to treatments (such as PARP inhibitors and DNA-damaging agents) as well as improved survival in newly diagnosed patients.1,2,15
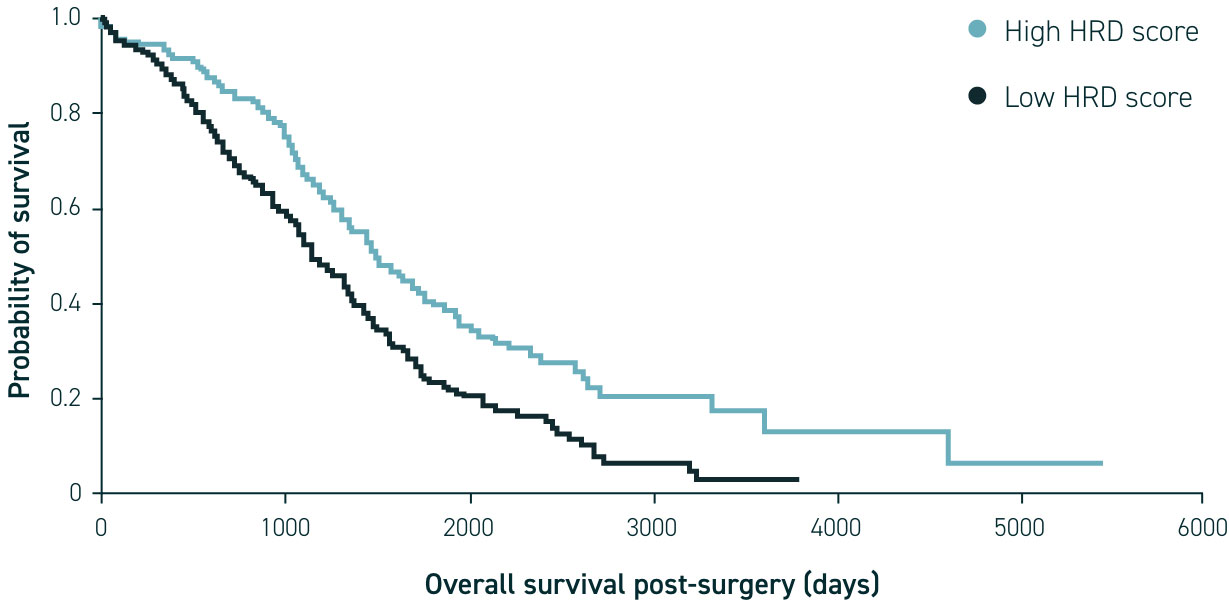
Identification of HRD status via genomic instability testing and BRCA mutation (BRCAm) testing using a sample of tumour tissue provides an opportunity for personalised or targeted treatment16–19
HRD testing identifies more patients who may be suitable for targeted therapies than BRCA testing alone1,2,20
Approximately 1 in 4 women with advanced ovarian cancer have a BRCA mutation, whereas ~1 in 2 have homologous recombination deficiency1,5,6
All women with a BRCA mutation are HRD-positive, but BRCA mutations are not the only cause of homologous recombination deficiency1
International guidelines increasingly recognise the value of testing for homologous recombination deficiency via BRCA mutation testing and/or genomic instability testing to inform treatment decisions21–24
aNCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
1. Konstantinopoulos PA, et al. Cancer Discov 2015;5:1137–1154; 2. Bonadio RRCC, et al. Clinics (Sao Paulo) 2018;73(suppl.1):e450s; 3. Audeh MW, et al. Lancet 2010;376:245–251; 4. Ledermann J, et al. Lancet Oncol 2014;15:852–861; 5. Ray-Coquard I, et al. Presented at European Society for Medical Oncology Annual Meeting 2019; 27th September – 1st October 2019; Barcelona, Spain; 6. González-Martin A, et al. N Engl J Med 2019;381:2391–2402; 7. O’Connor MJ. Mol Cell 2015;60:547–560; 8. Ferguson LR, et al. Semin Cancer Biol 2015;35(suppl.):S5–S24; 9. Peng G, et al. Nat Commun 2014;5:3361; 10. Hanahan D, Weinberg RA. Cell 2011;144:646–674; 11. Lord CJ, Ashworth A. Nature 2012;481:287–293; 12. Van Den Bosch M, et al. Biol Chem 2002;383(6):873–924; 13. Lieber MR, T Wilson TE. Cell 2010;142:496–496.e1; 14. Roy R, et al. Nat Rev Cancer 2012;12(1):68–78; 15. Abkevich V, et al. Br J Cancer 2012;107:1776–1782; 16. Moore K, et al. N Engl J Med 2018;379:2495–2505; 17. Coleman RL, et al. Lancet 2017;390(10106):1949–1961; 18. Mirza MR, et al. N Engl J Med 2016;375(22):2154–2164; 19. Ray-Coquard I, et al. N Engl J Med 2019;381:2416–2428; 20. Cancer Genome Atlas Research Network. Nature 2011;474(7353):609–615; 21. Miller RE, et al. Ann Oncol 2020;31(12):1606–1622; 22. Tew WP, et al. J Clin Oncol 2020;38(30):3468–3493; 23. Society of Gynecologic Oncology. SGO Clinical Practice Statement: Genetic Testing for Ovarian Cancer. Available at: https://www.sgo.org/resources/genetic-testing-for-ovarian-cancer/ (Accessed July 2021); 24. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Ovarian Cancer V.1.2021. © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Accessed [July 07, 2021]. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. To view the most recent and complete version of the guideline, go online to NCCN.org.